EXECUTIVE SUMMARY The CEDIT assessed the value and the impact of the device Radical-7™ (Masimo company) for non-invasive continuous monitoring of hemoglobin during and after surgery. Technical aspects: This device is composed of a sensor, a patient cable and a monitor. It enables the continuous monitoring of multiple physiological parameters, including hemoglobin, using finger plethysmography […]
STAR™ – radiofrequency ablation system for vertebral metastasis.
EXECUTIVE SUMMARY The CEDIT assessed the clinical value of the STAR™ (company D-FINE) radiofrequency ablation system intended to treat vertebral metastasis. Technical aspects: STAR device combines a bipolar directional flexible probe equipped with two thermocouples measuring per-process temperature, to an RF generator – and monitoring unit. According to the manufacturer, these features allow to consider […]

Aquapheresis – ultrafiltartion for congestive heart failure
EXECUTIVE SUMMARY The CEDIT assessed the value of the therapeutic method called « aquapheresis », process aiming to reduce in a non-pharmacological way the fluid overload of patients with congestive heart failure. Technical aspects: The term « ultrafiltration » designates the water extraction from the vascular system via a membrane, either by hemodialysis or by haemofiltration. The term « aquapheresis » […]

Antigermix – disinfection of endocavitary ultrasonography probes
EXECUTIVE SUMMARY The CEDIT assessed the value of ultraviolet rays UV-C (the Antigermix device) used for disinfection of endocavitary ultrasonography probes (endorectal and endovaginal), potential carriers for microbiological contaminations accross patients. Technical aspects: The Antigermix disinfection unit manufactured by Germitec uses ultraviolet rays with a wavelength of 254 nm generated by six low-pressure mercury gas-discharge […]

Surveillance peropératoire non invasive en continu de l’hémoglobinémie (dispositif Masimo Radical-7)
Consulter l’avis du CEDIT : Avis du CEDIT Consulter la présentation au CEDIT plénier : présentation monitorage Hb RESUME Le CEDIT a été saisi par l’AGEPS pour évaluer l’intérêt du dispositif Radical-7™ (entreprise Masimo) de monitorage continu non invasif de l’hémoglobinémie, pour une utilisation en per et/ou postopératoire. Aspects techniques : cet appareil est […]

Dispositif STAR™ pour l’ablation par radiofréquence des métastases vertébrales
Consulter l’avis du CEDIT : Avis du CEDIT Consulter la présentation au CEDIT plénier : présentation STAR RESUME Le CEDIT a été saisi par la COMEDIMS pour évaluer l’intérêt du dispositif STAR™ d’ablation par radiofréquence des métastases vertébrales (entreprise D-FINE). Aspects techniques : le dispositif Star associe une sonde bipolaire et courbable munie […]

Vivostat® fibrin sealant
View the INAHTA Brief EXECUTIVE SUMMARY The CEDIT, the hospital based HTA agency of the Paris University Hospital (AP-HP), has already assessed Vivostat® in 2006 and concluded at the time that the available data was insufficient to recommend its dissemination for clinical practice. However, in order to encourage the evaluation of innovative medical devices, the […]

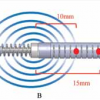
Magnetic growth rods for the scoliosis treatment in children
Visualise the INAHTA Brief EXECUTIVE SUMMARY The CEDIT assessed the impact of magnetic growth rods for the scoliosis treatment in children, in order to inform the decision making process of its adoption at the Paris University Hospital (AP-HP). Scoliosis is a sideways permanent curvature of the spine. The severe form, Early Onset Scoliosis (EOS), could […]
Chronologie
- juin 2016 (1)
- mars 2016 (2)
- février 2016 (1)
- juillet 2015 (2)
- juin 2015 (6)
- mai 2015 (8)
- avril 2015 (6)
- mars 2015 (1)
- mai 2014 (8)
- janvier 2014 (6)
- décembre 2013 (1)
- octobre 2013 (1)
- août 2013 (1)
- juin 2013 (4)
- mai 2013 (1)
- décembre 2012 (23)
- juillet 2012 (1)
- avril 2012 (1)
- janvier 2012 (1)
